Abstract
Introduction
Peripheral T-cell lymphoma (PTCL) is a group of hematological malignancies originating from mature T/NK cells. Most of the subtypes are associated with aggressive clinical features and dismal outcomes. Routine first-line chemotherapy has low efficiency and a high recurrence rate, so there is an urgent need for new drugs. Monotherapy or combination therapy of epigenetic inhibitors have been shown to be effective in several hematologic malignancies. Here, we report the interim efficacy of an epigenetic priming regimen with azacytidine and chidamide prior to salvage chemotherapy for relapsed or refractory (R/R) PTCL.
Methods
The prospective phase II study (ChiCTR2000037232) enrolled pts were pathologically confirmed T/NK cell non-Hodgkin's lymphoma with at least one imaging measurable lesion. Pts needed to have received at least one systemic chemotherapy regimen including hematopoietic stem cell transplantation, radiotherapy, or a single epigenetic drug. Pts received AZA hypodermically at a dose of 100 mg on days 1 to 7, chidamide of 20 mg orally twice per week; the combined chemotherapy regimens included but were not limited to GemOx (gemcitabine, oxaliplatin); CPT (cyclophosphamide, prednisone, thalidomide) , etc. Treatment was performed for up to eight cycles of each 21 days. Pts who achieved partial response (PR) and better remission began maintenance therapy every two months with double epigenetic inhibitors for two years. The trial aimed to explore the efficacy and safety of AZA and chidamide combined chemotherapy in the treatment of R/R PTCL. The primary objective was investigator-assessed best overall response rate (ORR). Secondary objectives included duration of response (DOR), complete response rate (CRR), progression-free survival (PFS), overall survival (OS), and safety profiles.
Results
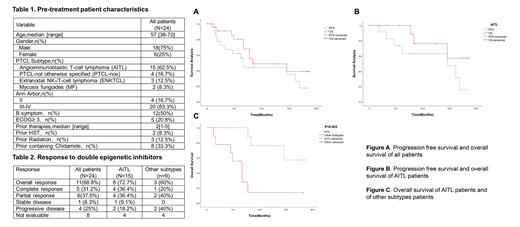
A total of 24 pts have been enrolled, baseline characteristics are shown in Table 1. Pathological subtypes included angioimmunoblastic T-cell lymphoma(AITL, n=15), PTCL-not otherwise specified (PTCL-NOS, n=4), extranodal NK/T-cell lymphoma (ENKTCL, n=3) and mycosis fungoides(MF, n=2). The median age was 57 (range,38-72) years with male predominance. Ann Arbor Classification ≥ stage III in 20 pts. Twelve pts had B symptoms at the time of diagnosis, five pts had performance status ≥ 3 before treatment. The median number of previous systemic treatment regimens was two. Autologous hematopoietic stem cell transplantation in two pts, radiation in three pts and prior treatments containing chidamide in eight pts. At the time of data cutoff, the median number of treatments for all pts was four cycles (range,1-13). Among 16 pts evaluable for response, the best ORR was 68.8% (11/16) with five pts achieved CR, six achieved PR. In subgroup analysis, eleven AITL pts achieved an objective response. The best ORR was 72.7% (8/11) with four pts attained CR, four attained PR (Table 2). The median follow-up was 12.4 (range, 0.1-18.7) months. For all pts, the median PFS was 6.7 months (95% CI,5.8-7.6), the median OS was 8.4 months (95% CI,0.0-18.3) (Figure 1). And the median DOR was 10.2 months (95% CI,4.9-15.5). For AITL pts, the median PFS was 14.6 months (95% CI,3.6-25.6), and the median OS was not reached (Figure 2). The OS between AITL and other subtypes pts was statistically significant (1-year OS: 76.2% vs 13.9%; p=0.003, Figure 3). Almost all pts had experienced at least one adverse event (AE). The most common grade 3 or 4 AEs were anemia, leukopenia, neutropenia, thrombocytopenia, and infections.
Conclusions
Epigenetic priming regimen with azacitidine plus chidamide with salvage chemotherapy is effective and tolerable. The best ORR of all enrolled pts with AITL were 68.8% and 72.7%, respectively. Compare to other subtypes, patients with AITL subtype benefit more obviously from our regimen with durable remission. And further studies will focus on patients with AITL and follicular helper T-cell originated.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal